We are proud to announce for the first time the…
A LEADING ROLE IN THE PHARMA SECTOR.
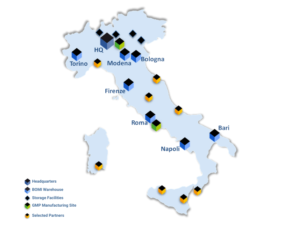
Following the recent acquisition of Logifarma, a player specialized in pharmaceutical logistics located in Pomezia (RM), BOMI Group officializes the creation of the Pharma Italia Business Unit and establishes itself as a logistics operator able to manage directly storage and transport of pharmaceutical products throughout the country.
The B.U. Farma is structured on two logistic poles, the first one in Soresina (CR) and the second one in Pomezia, near the pharmaceutical production centers of Lazio, covering a total area of about 40.000 square meters.
Bomi Group’s B.U. Pharma Italy provides its customers with a GMP authorized area dedicated to the management of product inbound, three AIFA authorized pharmaceutical laboratories (with about 40 million packs reprocessed each year), a production warehouse with storage activities for API raw materials, import management for semi-finished and raw materials (API), an area dedicated to the preparation of the finished product.
We have the following licenses:
- HOLDING PHARMACEUTICAL PRODUCTS FOR HUMAN USE DGLS 219/2006;
- NARCOTICS AND DRUGS AUTHORIZATION DPR 309/1990;
- VETERINARY AUTHORIZATION DGLS 193/2006;
- API WAREHOUSING AND DISTRIBUTION AUTHORISATION;
- FOOD SUPPLEMENTS AND INGREDIENTS NOTIFICATION.
In addition to the two Pharma sites and the Headquarters located in Spino d’Adda (CR), the Bomi Group‘s Italy network is articulated through 8 other transport hubs located from North to South Italy which transit products to all the destinations.
Through the BOMI HEALTH CARRIER fleet, consisting of about 300 insulated and refrigerated vehicles, Bomi manages the transport for its customers in compliance with GDP standards. In 2019 Bomi completed over 1,200,000 deliveries to: hospitals, wholesalers, pharmacies, parapharmacies and home patients.
For any information on the Italy network: commerciale@bomigroup.com